Product name
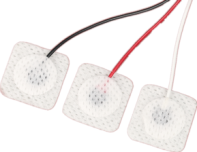
Wired electrode patches for newborns
Super soft secure fit | Medical grade allergenic colloids | Adapt to NICU multi-parameter monitoring
1. Product Overview
Material Composition:
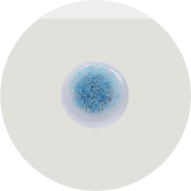
Conductive layer: Chlorine-free solid hydrogel (Ag/AgCl-Free, impedance ≤ 3kΩ, ISO 10993-10 sensitization test)
Base material: ultra-thin hydrocolloid dressing (thickness 0.02mm, air permeability ≥ 5000g/m²/24h)
Wire: Medical silicone coated flexible cable (length 30cm/50cm optional, tensile strength ≥ 15N)
Scope of application:
Neonatal electrocardiogram (ECG), electroencephalogram (EEG) monitoring, NICU multi-parameter monitoring, and preterm infant vital signs tracking.
Applicable scenarios: delivery room, neonatal intensive care unit (NICU), transport incubator
Second, the core advantages
1. Neonatal research design
Extremely gentle: chlorine-free gel + hydrocolloid substrate, passed the neonatal skin irritation test (no erythema/edema after 72 hours of application).
Mini size: 15mm electrode diameter (designed for premature infants), optional 20mm standard version, to avoid blocking fontanelles and sensitive parts.
Anti-shedding guarantee: medical silicone wire + 360° annular adhesive, withstand incubator humidity (90%RH) and body position movement (tensile test≥8N).
2. Customized service (stand-alone module)
Customized projects Options
Wire length: 30cm (monitoring in the incubator), 50cm (open rescue table), 80cm (transfer monitoring)
Electrode color: transparent (does not affect jaundice phototherapy), fluorescent (fast positioning in low-light environment)
Interface Type: Snap type (Philips/GE compatible), pin type (Mindray/Libon adaptation), magnetic quick connect (wireless transmitter integration)
Packaging form: single piece of independent sterilization (ethylene oxide), 5 pieces of tear-off package (high-frequency use of first aid), customized department label
3. Global Safety Certification (Certification Icon Wall)
Special certifications: ISO 12485-2016 (safety standard for neonatal medical devices), EN 14375 child safety packaging
Basic certification: CE MDR, FDA 510 (k), China NMPAII
Biological testing: validated by OECD 439 in vitro model of skin irritation/corrosiveness
3. Technical parameter table
Project parameters
Contact Impedance: ≤3kΩ (10Hz-150Hz, AAMI EC11:1991 standard)
Adhesion 0.5-0.8N/cm² (simulated neonatal skin, 72-hour test)
Wire bending life ≥ 5000 times (radius of curvature 10mm, ASTM D5276 test)
Sterilization method: Ethylene oxide sterilization (residue< 4 μg/cm², ISO 10993-7)
Environmental Resistance: Temperature 25-40°C, Humidity 20-95%RH (Measured in Incubator Environment)
Fourth, the use of the scene diagram
NICU monitoring: the chest wall of premature infants is attached to electrodes, and the wires are connected to multi-parameter monitors
First aid in the delivery room: The neonatal resuscitation table is quickly attached, and the heart rate/respiratory waveform is displayed synchronously
Family monitoring: High-risk children use wireless models at home, and parents can view real-time data through the APP

ECG patches have CE and other multi-national certifications, can be sold worldwide, and can accept customized services, including but not limited to shape, material, brand and box size, etc. We are committed to meeting the needs of our customers






 12 years
China
12 years
China




rosetyler –
Bibendum at varius vel pharetra vel turpis. Egestas dui id ornare arcu odio ut sem nulla. Amet porttitor eget dolor morbi non. At ultrices mi tempus imperdiet nulla malesuada. Pretium vulputate sapien nec sagittis aliquam malesuada bibendum. Bibendum arcu vitae elementum curabitur vitae. Facilisi cras fermentum odio eu feugiat pretium nibh.
admin –
Lorem Ipsum is simply dummy text of the printing and typesetting industry.