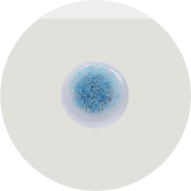
Product name
Single-use liquid wet gel electrode patches
High conductivity hydrogel | Support customized formulations | Full coverage of global export qualifications
1. Product Overview
Material Composition:
Conductive layer: liquid silver chloride hydrogel (Ag/AgCl, with moisturizing factor)
Base material: Medical PU film (thickness 0.08mm±0.01mm)
Backing: Silicone oil release liner (anti-sticking and anti-oxidation)
Scope of application:
High-precision scenarios such as Holter, sleep apnea monitoring, and long-term bedridden patient monitoring.
Suitable for people: for long-term monitoring of adults (can be customized for motion monitoring and anti-falling out)
Second, the core advantages
1. Liquid wet glue performance advantages
Ultra-low impedance: liquid gel fills skin microlines, and the contact impedance is ≤ 1.5kΩ (30% better than ANSI/AAMI EC12 standard).
Long-term moisturizing: 72 hours of continuous conductivity without drying out (measured in 25°C/60%RH environment), suitable for 24-hour Holter monitoring.
Residue-free experience: water-soluble adhesive with no gum residue after removal, reducing the risk of skin irritation (ISO 10993 biocompatibility tested).
2. Customized service (stand-alone module)
Customized projects Options
Electrically conductive adhesive formulation: Standard type (silver chloride), hypoallergenic type (chlorine-free formula), high viscosity type (motion anti-shedding)
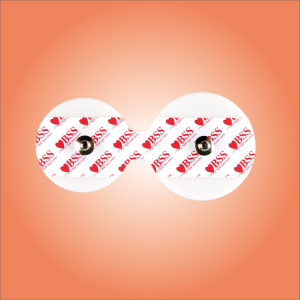
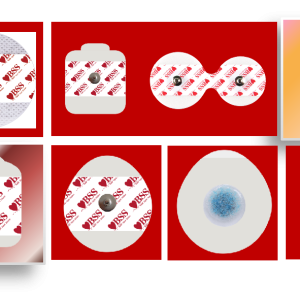
Electrode shape: round (regular), rectangular (for thoracic leads), special-shaped (CT/MRI compatible anti-counterfeit models)
Packing: Monolithic aluminum foil sealing (anti-oxidation), 10 pieces/bag (economy), integrated packaging with wires (plug and play)
Identification system Multilingual instructions, UDI code printing, patient skin type warning labels (e.g. sensitive skin red)
3. Global Export Qualification (Certification Icon Wall)
Core certifications: CE MDR 2017/745, FDA 510(k)(KXXXXXX), ISO 13485:2016
Regional expansion: Japan’s PMDA, Brazil’s ANVISA, Australia’s TGA (support for targeted market supplements)
Environmental compliance: RoHS 2.0, REACH SVHC no hazardous substance declaration
3. Technical parameter table
Project parameters
Conductive adhesive moisture content 65%±5% (to ensure the balance of conductivity and viscosity)
Skin Contact Impedance: ≤1.2kΩ (10Hz-1kHz band, ASTM F2503)
Adhesion durability ≥72 hours (measured peeling force in dry environment≥ 0.8N/cm²)
Sterilization method: Gamma sterilization (25kGy, optional EO sterilization)
Storage conditions: 10-30°C in a cool and dry environment, avoid freezing (gel phase change temperature≥5°C)
ECG patches have CE and other multi-national certifications, can be sold worldwide, and can accept customized services, including but not limited to shape, material, brand and box size, etc. We are committed to meeting the needs of our customers







 12 years
China
12 years
China





rosetyler –
Lorem ipsum dolor sit amet consectetur adipiscing elit. Est velit egestas dui id ornare arcu odio ut. Nulla pellentesque dignissim enim sit amet venenatis. Malesuada fames ac turpis egestas. Nibh praesent tristique magna sit amet purus. Arcu felis bibendum ut tristique et egestas quis ipsum.